Loading...
Found 345 Barrett's Esophagus trials
A listing of Barrett's Esophagus medical research trials actively recruiting patient volunteers. Search for closest city to find more detailed information on a research study in your area.

This is a pilot study. Subjects with primary soft tissue sarcoma of the extremities may be eligible for this study. Subjects may participate in this study if they are at least 18 years of age. Most participants will be receiving care at the clinical practices of the University of Pennsylvania …

This study aims to compare IBD patients with healthy controls to obtain a systems-level view of immune system perturbation by IBD. This intervention (anti-TNF therapy) will be intended to treat IBD but will also represent a major, broad immune system perturbation. Although the response of the patient in terms of …

This research study will test the performance of new magnetic resonance imaging techniques at 7.0 Tesla. These new methods have already been tested in model systems and need to be tried on human subjects prior to their applications in determining important substances in the whole brain. . This study is …
The study will enroll cognitively unimpaired individuals who are between the ages of 60-75 years with at least one APOE4 allele (HMs or HTs) and, if HTs, with evidence of elevated brain amyloid. Cognitively unimpaired subjects defined as having a Mini-Mental State Examination (MMSE) total score greater than or equal …

BAN2401-G000-301 (Study 301) is an 18-month treatment (Core Study), multicenter, double-blind, placebo-controlled, parallel-group study in subjects with EAD (mild cognitive impairment [MCI] due to AD with intermediate likelihood/Prodromal AD or mild AD dementia) with confirmed amyloid pathology indicated by either positive amyloid load confirmed by amyloid PET assessment or CSF …
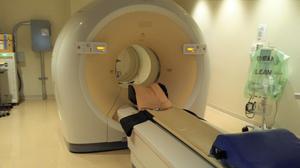
Patients with recurrent or metastatic ER+ breast cancer, who have failed prior endocrine therapy may be eligible for this study. Patients may participate in this study, if they are at least 18 years of age, most participants will be receiving care at the clinical practices of the University of Pennsylvania. …
Women with known or suspected epithelial ovarian, fallopian tube, or primary peritoneal cancer may be eligible for this study. Patients may participate in this study if they are at least 18 years of age. Up to 30 evaluable subjects who have been recommended to start systemic therapy as part of …
The purpose of this study is to learn how to dialysis doctors can treat dialysis patients infected with Hepatitis C themselves. Hepatitis C infection is common in hemodialysis patients. There are new medications, such as Zepatier, which can cure hepatitis C in > 95% of patients. Zepatier is particularly good …

SGM-101 is a fluorochrome-labeled anti-carcino-embryonic antigen (CEA) monoclonal antibody. What this means is that part of SGM-101 is a chemical that is attracted to and will attach to the cells of your cancer. Another part of SGM-101 is a chemical that will glow when infrared light is shined on it. …

This is a multicenter, non-blinded, prospective observational cohort study of 1000 patients enrolled in an AlloSure testing registry, including 300 patients at centers with planned renal surveillance biopsies at 12 months post-transplantation. The other 700 patients will be from centers that do not perform protocol surveillance biopsies. This registry study …